Project 3: Combined quantum mechanics and molecular mechanics study of interprotein electron transfer
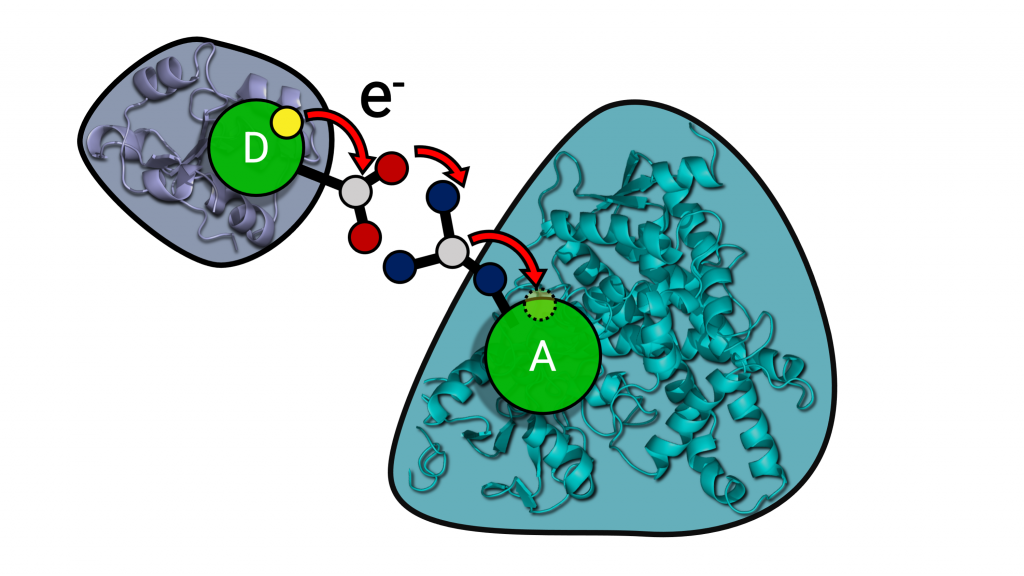
Electron transfer processes play an important role in many biological processes, such as photosynthesis, cellular respiration and drug metabolism. In this project, we study the transfer of electrons required for catalysis by cytochrome P450 (CYP) enzymes. These are ubiquitous enzymes with key roles in steroid synthesis and drug metabolism that are also of interest as biocatalysts. Two electrons are provided by partner redox proteins and in some cases, electron transfer can be the rate-limiting step of the reaction. The Wade group (HITS) has developed a multiscale approach combining coarse-grained and atomic-detail Brownian and molecular dynamics methods to build and simulate models of CYP-redox protein complexes in phospholipid bilayers and compute electron transfer pathways using an empirical approach [Mukherjee et al., 2021]. The Elstner group (KIT) has developed multiscale QM/MM approaches based on the semi-empirical DFTB method for studying electron transfer in enzymes [Lüdemann et al. 2015]. In this collaborative project, we will combine classical molecular mechanics and quantum mechanical simulation approaches, as well as machine learning, to identify the determinants of electron transfer rates in different CYP isoforms and to understand how mutations affect electron transfer.
References:
Mukherjee et al. (2021). An electron transfer competent structural ensemble of membrane-bound cytochrome P450 1A1 and cytochrome P450 oxidoreductase Communications biology 4:55
Lüdemann et al. (2015). Solvent driving force ensures fast formation of a persistent and well-separated radical pair in plant cryptochrome, J Am Chem Soc 137:1147–1156.
Team

Prof. Dr. Rebecca Wade
Principal Investigator and Deputy Scientific Director (HITS)
Phone: +49 6221 533 247
More Information
Prof. Dr. Marcus Elstner
Principal Investigator (Karlsruhe Institute of Technology)
Phone: +49 721 60845705
More Information