Force-triggered blood coagulation
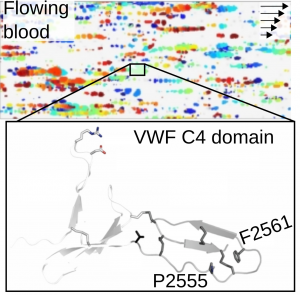
Von Willebrand factor (VWF) is a giant multidomain extracellular blood protein in charge of a key adhesive process during hemostasis: the recruitment of platelets at sites of injury and further formation of a plug that prevents bleeding. VWF carries out this function in response to mechanical stimuli originated by the shear of the flowing blood (top figure: platelet-VWF rolling aggregates in flowing blood).
We employ a multidisciplinary approach, that combines computer simulations with structural biology, biophysical and biochemical experiments, along with clinical assays to understand the molecular principles governing VWF function and its alterations leading to bleeding diseases.
Our research seeks to elucidate the effect of disulfide bonds on the structural stability and function of VWF and to uncover how disease-related mutations alter these two. In particular, we have recently revealed how the mutations Pro2555Arg and Phe2561Tyr in the VWF C4 domain alter VWF function resulting in unwanted platelet aggregation (bottom figure). With this research we ultimately seek to delineate molecular principles that guide the manipulation of VWF with clinical and nanotechnological purposes. Figure adapted from Huck et al. Thrombosis and Haemostasis DOI: 10.1055/a-1344-4405 (2020).
More information:
Volker Huck, Po-chia Chen, Emma-Ruoqi Xu, Alexander Tischer, Ulrike Klemm, Camilo Aponte-Santamaría, Christian Mess, Tobias Obser, Fabian Kutzki, Gesa König, Cecile Denis, Frauke Gräter, Matthias Wilmanns, Matthew Auton, Stefan Werner Schneider, Reinhard Schneppenheim, Janosch Hennig, Maria Alexandra Brehm (2021). Gain-of-function variant p.Pro2555Arg of von Willebrand factor increases aggregate size through altering stem dynamics. Thrombosis and Haemostasis DOI: 10.1055/a-1344-4405.
Xu E, Bülow Sv, Chen P, Lenting PJ, Kolšek K, Aponte-Santamaría C, Simon B, Foot J, Obser T, Schneppenheim R, Gräter F, Denis CV, Wilmanns M, Hennig J (2019). Structure and dynamics of the platelet integrin-binding C4 domain of von Willebrand factor, Blood 133(4):366-376.