Revealing the behaviour of von Willebrandt Factor through experiments and simulations
Dr. Camilo Aponte-Santamaria has recently published three papers revealing the behaviour of the von Willebrandt factor.
The work describes a new force-sensory mechanism for VWF-platelet binding, which addresses the inactivation of VWF by shielding of its adhesion sites, combining molecular dynamics (MD) simulations, atomic force microscopy (AFM), and microfluidic experiments. The simulations demonstrate that the VWF A2 domain (green) targets a specific region at the VWF A1 domain (blue), corresponding to the binding site of the platelet glycoprotein Ibα (GPIbα) receptor (red), thereby causing its blockage. This implies autoinhibition of the VWF for the binding of platelets mediated by the A1-A2 protein-protein interaction. A stretching force dissociated the A1A2 complex before unfolding of A2, ensuring VWF platelet-binding activation before unfolding-mediated proteolytic cleavage. Microfluidic experiments with an A2-deletion VWF mutant resulted in increased platelet binding, corroborating the key autoinhibitory role of the A2 domain within VWF multimers. Overall, autoinhibition of VWF mediated by force-dependent interdomain interactions offers the molecular basis for the shear-sensitive growth of VWF-platelet aggregates, and might be similarly involved in shear-induced VWF self-aggregation and other force-sensing functions in hemostasis.
You can read more about this here.
Additionally Dr. Aponte-Santamaria and his collaborators also describe the role of some mutations on the ability of the protein to form dimers. The simulations predict that VWF mutations, abolishing its dimerization, induce structural changes at the dimer-linking CK domain.
You can read more about this here.
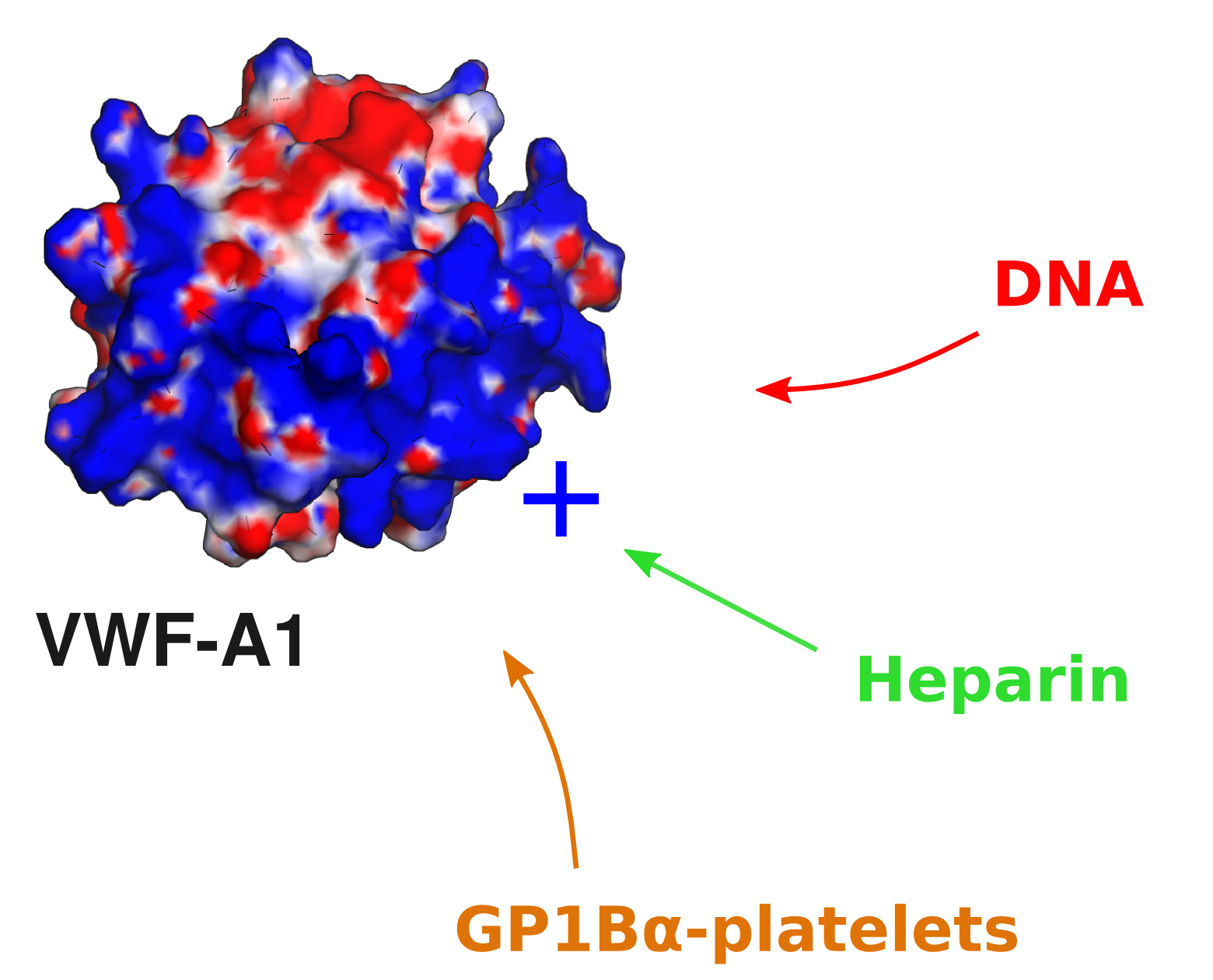
Recently, the potential role of vWF in binding different cellular components were also investigated. Experimental collaborators (from the Schneider lab in Mannheim) demonstrated that the von Willebrand factor (VWF) interacts with DNA, an interaction with implications under inflammatory processes. The simulations complemented these findings suggesting that a positively charged region at the VWF A1 domain serves as the binding site for the -negatively charged- DNA, and that GP1Ba-platelets complexes and heparin compete with DNA for this binding site.
Information about this project can be found here.
Über das HITS
Das HITS (Heidelberger Institut für Theoretische Studien) wurde 2010 von dem Physiker und SAP-Mitbegründer Klaus Tschira (1940-2015) und der Klaus Tschira Stiftung als privates, gemeinnütziges Forschungsinstitut gegründet. Es betreibt Grundlagenforschung in den Naturwissenschaften, der Mathematik und der Informatik. Zu den Hauptforschungsrichtungen zählen komplexe Simulationen auf verschiedenen Skalen, Datenwissenschaft und -analyse sowie die Entwicklung rechnergestützter Tools für die Forschung. Die Anwendungsfelder reichen von der Molekularbiologie bis zur Astrophysik. Ein wesentliches Merkmal des Instituts ist die Interdisziplinarität, die in zahlreichen gruppen- und disziplinübergreifenden Projekten umgesetzt wird. Die Grundfinanzierung des HITS wird von der Klaus Tschira Stiftung bereitgestellt.