Mechanosensing ability of Focal Adhesion Kinase
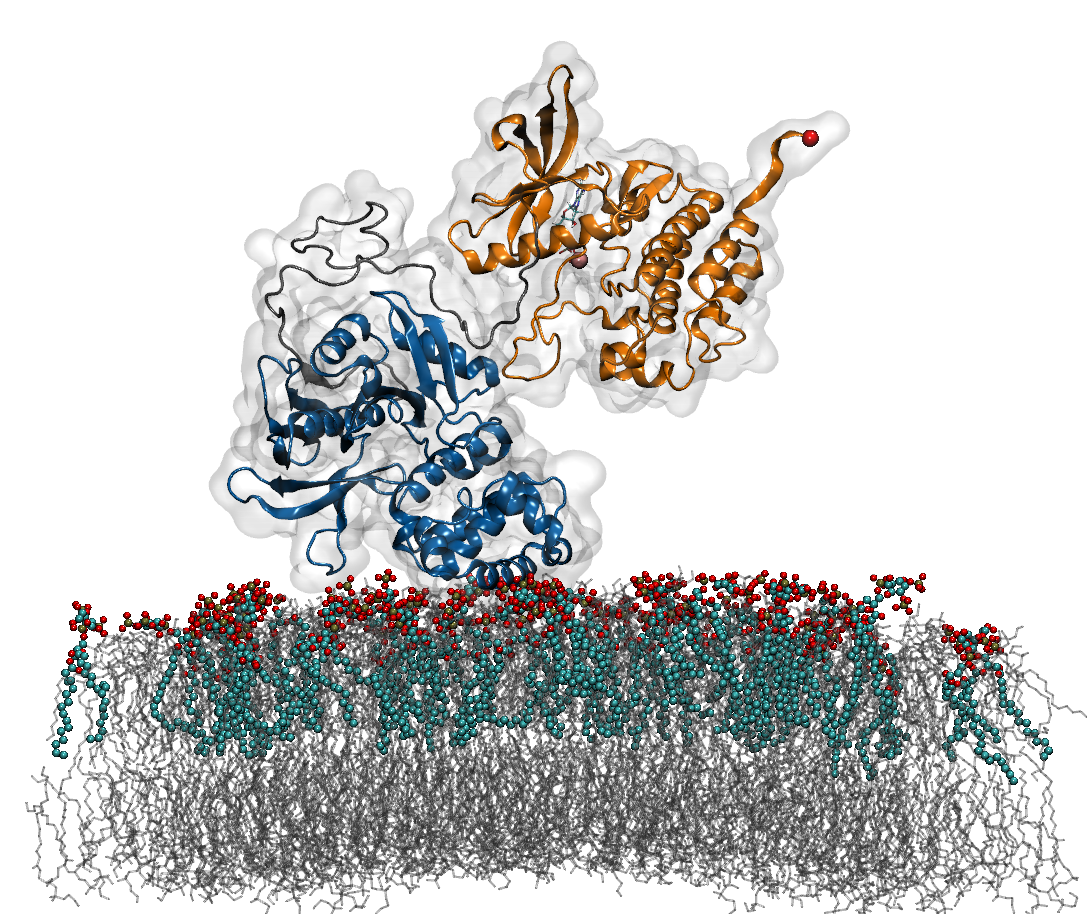
Mechanosensing plays a decisive role at focal adhesions (Fas), sites at which external forces are integrated into cellular signaling pathways, thereby deciding upon vital processes such as cell proliferation and differentiation. During the past decade, an increasing number of FAs molecules has been recognized as a force-sensitive enzyme involved in the translation of mechanical force from extracellular matrix into gene expression in the cell nucleus. Based on the results of a combined approach of Molecular Dynamic (MD) and biochemical network simulations, we suggest Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, as a mechano-sensing enzyme in FAs.
A growing body of evidence shows that tensile-stress acting on cells leads to an increase in FAK activation, however, the mechanism of underlying FAK activation is still lacking. According to the crystal structure of FAK, the major mechanism of FAK activity is an intramolecular autoinhibitory interaction between two of its domain – the central catalytic and N-terminal FERM domains. Recent study [1] shows that interactions of FAK with phosphoinositide phosphatidylinsositol-4,5-bis-phosphate (PIP2) are required to activate FAK. Thus, the ligand induced conformational changes in FAK have been studied with MD simulations and Force Distribution analysis (FDA), which provides the evidence that an external force as an additional stimulus is required for FAK activation. Following this result, extensive Force Probe Molecular Dynamics (FPMD) simulations with varying loading rates, pulling directions and membrane PIP2 concentrations are carried out to examine the propagation of tensile forces from the membrane through the PIP2 binding site of the FERM domain and from the cytoskeleton-anchored FAT domain, activate FAK by relieving the occlusion of the central phosphorylation site of FAK (Tyr576/577) . The force-dependent FAK kinetics based on extrapolated MD simulations are further implemented as parameters to establish a mechano-biochemical network model to provide direct insight into the function of FAK as a mechano-enzyme in the regulation of the Ras activation and other downstream signaling pathways.
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.