An unstructured protein as a dog-leash
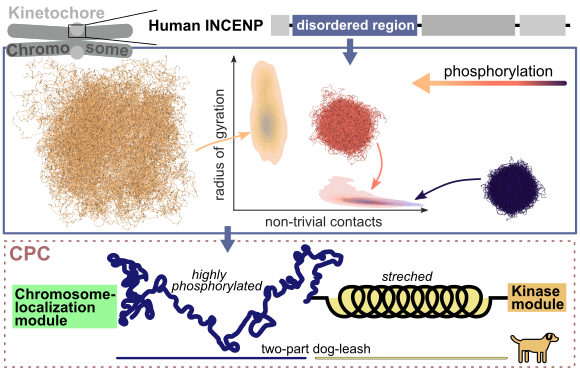
A key process of all cellular life is cell division, in which the genetic material of one cell is duplicated and divided into two daughter cells. The duplicated chromosomes align in the cell center and are positioned there by cellular filaments, the microtubules. This chromosome-microtubule binding is established and carefully fine-tuned by large protein complexes, the so-called kinetochores. Wrongly attached chromosomes must be first disengaged and reattached to ensure equal separation of the genetic material. One essential player in this error correction mechanism is the inner centromere protein (INCENP), which contains a long, unstructured region (IDR, intrinsically disordered region). The function of this domain, which can be highly modified by phosphorylation, is widely unknown.
Using molecular dynamics simulations, both on the atomic scale as well as on a coarser level, researchers from the MBM group investigated the effect of phosphorylation on this domain. They found that phosphorylation enlarges the dimensions of the IDR mainly by introducing negative charges. With phosphorylation, the IDR shifts from collapsed conformations, characterized by many internal contacts, to weakly interacting stretched states. In this way, phosphorylation also tunes the cohesiveness of several INCENP molecules interacting with each other. These data suggest that the INCENP IDR might act as a phosphorylation-tuned and length-variable component within the so called “dog-leash model”, which was proposed by previous studies (Krenn et al. 2015). Here, the kinase Aurora B, responsible for detaching wrong chromosomes, is regulated by a “dog-leash”, which controls the distance of Aurora B to target molecules. Thus, phosphorylation of the INCENP IDR might be one way to determine the action radius of Aurora B.
Check out the paper: Martin, Aponte-Santamaría, et al. “Phosphorylation tunes elongation propensity and cohesiveness of INCENP’s intrinsically disordered region”. J Mol Biol. 434: 167387 (2022).
Additional reference: Krenn, Veronica, Musacchio, Andrea. “The aurora b kinase in chromosome bi-orientation and spindle checkpoint signaling.” Front. Oncol. 5, 225. (2015).
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.