Revealing the behaviour of von Willebrandt Factor through experiments and simulations
Dr. Camilo Aponte-Santamaria has recently published three papers revealing the behaviour of the von Willebrandt factor.
The work describes a new force-sensory mechanism for VWF-platelet binding, which addresses the inactivation of VWF by shielding of its adhesion sites, combining molecular dynamics (MD) simulations, atomic force microscopy (AFM), and microfluidic experiments. The simulations demonstrate that the VWF A2 domain (green) targets a specific region at the VWF A1 domain (blue), corresponding to the binding site of the platelet glycoprotein Ibα (GPIbα) receptor (red), thereby causing its blockage. This implies autoinhibition of the VWF for the binding of platelets mediated by the A1-A2 protein-protein interaction. A stretching force dissociated the A1A2 complex before unfolding of A2, ensuring VWF platelet-binding activation before unfolding-mediated proteolytic cleavage. Microfluidic experiments with an A2-deletion VWF mutant resulted in increased platelet binding, corroborating the key autoinhibitory role of the A2 domain within VWF multimers. Overall, autoinhibition of VWF mediated by force-dependent interdomain interactions offers the molecular basis for the shear-sensitive growth of VWF-platelet aggregates, and might be similarly involved in shear-induced VWF self-aggregation and other force-sensing functions in hemostasis.
You can read more about this here.
Additionally Dr. Aponte-Santamaria and his collaborators also describe the role of some mutations on the ability of the protein to form dimers. The simulations predict that VWF mutations, abolishing its dimerization, induce structural changes at the dimer-linking CK domain.
You can read more about this here.
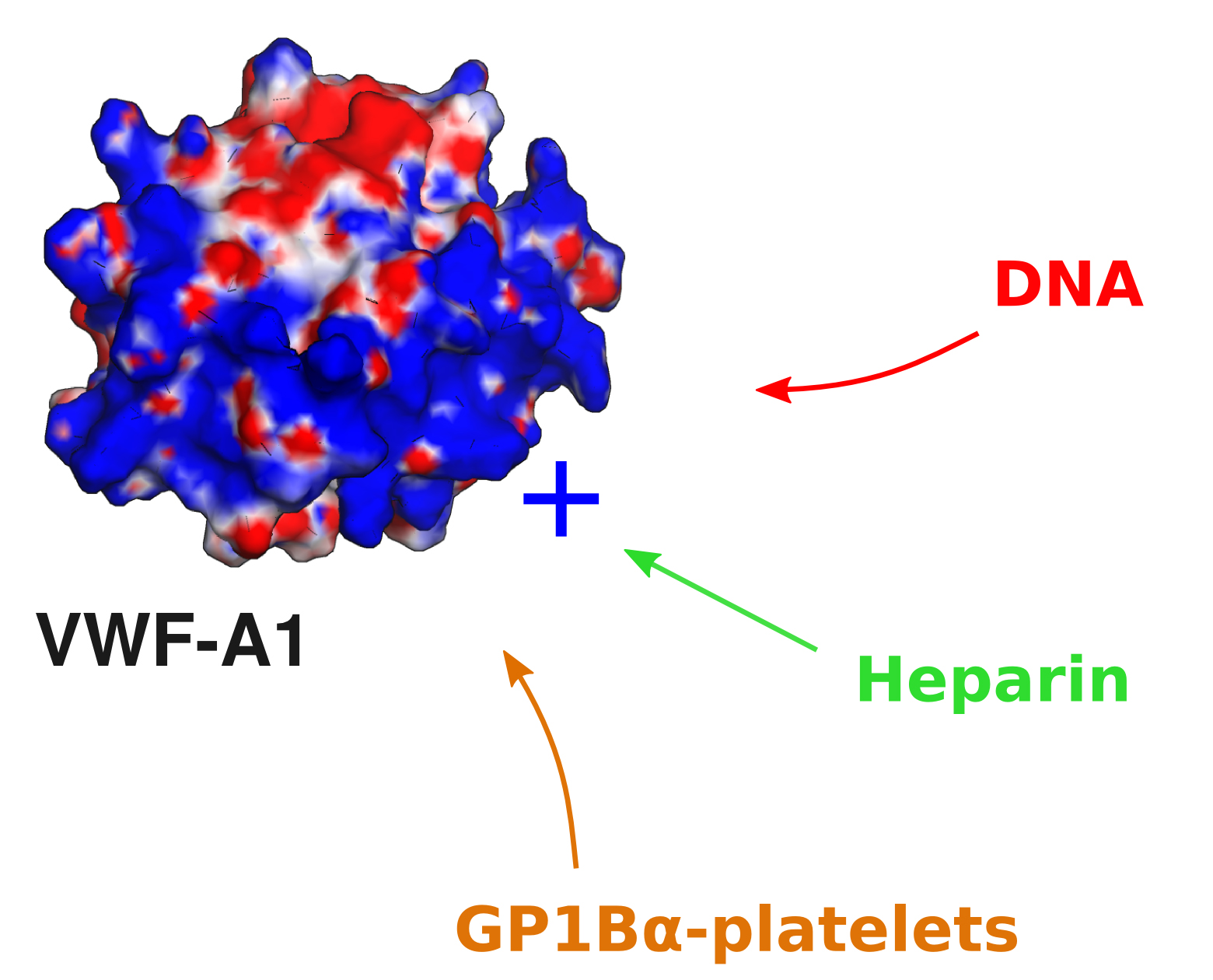
Recently, the potential role of vWF in binding different cellular components were also investigated. Experimental collaborators (from the Schneider lab in Mannheim) demonstrated that the von Willebrand factor (VWF) interacts with DNA, an interaction with implications under inflammatory processes. The simulations complemented these findings suggesting that a positively charged region at the VWF A1 domain serves as the binding site for the -negatively charged- DNA, and that GP1Ba-platelets complexes and heparin compete with DNA for this binding site.
Information about this project can be found here.
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.